 Original Article
Original Article - Evaluation of Efficacy and Safety of Ticagrelor Compared to Clopidogrel in East Asian Patients with Acute Coronary Syndrome: A Systematic Review and Meta-analysis
Bo Hee Lee and Yun Jeong Lee*
College of Pharmacy, Dankook University, Cheonan, 31116, Republic of Korea
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Dual antiplatelet therapy with aspirin and a P2Y12 inhibitor is the standard of therapy in patients with acute coronary syndrome (ACS). Ticagrelor is a newer oral P2Y12 inhibitor with favorable efficacy and safety outcomes. However, as there are limited and inconsistent findings on the use of ticagrelor compared to clopidogrel in East Asian patients with ACS, we aimed to evaluate the efficacy and safety of ticagrelor compared to clopidogrel by evaluating previous studies conducted in East Asians. A systematic search on studies comparing ticagrelor to clopidogrel in East Asian ACS patients was performed through PubMed, Embase, and Cochrane library by two independent reviewers. A total of 10 studies were included in the meta-analysis. Ticagrelor as compared with clopidogrel significantly reduced the rate of myocardial infarction, stroke, or cardiovascular death in our meta-analysis of the efficacy endpoint. For major bleeding, no significant differences were observed between the two groups. However, for minor bleeding, ticagrelor had significantly increased incidence compared to clopidogrel. In conclusion, in East Asian patients with ACS, ticagrelor can be used with better efficacy and similar incidence of major bleeding compared to clopidogrel, but minor bleeding occurred more frequently
Keywords: acute coronary syndrome, ticagrelor, clopidogrel, East Asians
Acute coronary syndrome (ACS) is a sudden condition of imbalance between myocardial oxygen demand and supply leading to myocardial ischemic states.1) It is initiated by an atheromatous plaque disruption or erosion. Involvement of lipid, foam cells and smooth muscle could promote a thrombus formation. By this process, it induces ischemic state which can be fatal. Acute coronary syndrome could be classified into unstable angina (UA), non-ST-segment elevation myocardial infarction (NSTEMI), and ST-segment elevation myocardial infarction (STEMI). Additionally, UA and NSTEMI are classified as non-ST-segment elevation acute coronary syndrome (NSTE-ACS). Morbidity and mortality of ACS have increased considerably worldwide. According to the World Health Organization (WHO), in 2016, the estimated number of deaths from cardiovascular diseases was 17.9 million.2) Likewise, in East Asians, cardiovascular death is a major issue and leading cause of death.3)
There are various therapeutic options for prevention of abnormal blood clot development or blockage of blood vessels. The American College of Cardiology and American Heart Association (ACC/AHA) recommend dual antiplatelet therapy (DAPT) as standard of therapy for ACS patients. Dual antiplatelet therapy contains an adenosine diphosphate (ADP) P2Y12 receptor inhibitor and aspirin.4) The P2Y12 receptor antagonists consist of ticlopidine, clopidogrel, prasugrel, ticagrelor, cangrelor and elionogrel.5) Among them, we focused on clopidogrel and ticagrelor.
Clopidogrel and ticagrelor have similar but different pharmacological mechanisms. Clopidogrel is an oral prodrug which irreversibly inhibits P2Y12 receptor, an ADP chemoreceptor on platelet cell membrane.6-7) It is primarily metabolized by CYP2C19 to an active metabolite, and it has somewhat of a slower activity compared to ticagrelor. In Caucasians, the loss-of-function CYP2C19*3 allele presents very rarely (2-5%), but in East Asians, it occurs more frequently at a rate of 13-23%.8-9) Such difference in the occurrence of genetic polymorphism could contribute to the response rate of clopidogrel. On the other hand, ticagrelor is an active moiety and it has faster onset and offset than clopidogrel.10-11)
In a large, global clinical trial, PLATelet inhibition and patient Outcomes (PLATO), it has been shown that ticagrelor significantly reduces cardiovascular related events and all-cause death with a similar rate in major bleeding events compared with clopidogrel.6) However, only 6% of Asians were included in PLATO. Another trial conducted in East Asians, PHILO, observed that event rates of primary efficacy endpoint of composite of myocardial infarction (MI), stroke an d death from vascular causes and primary safety endpoint of time to first occurrence of any major bleeding event in ticagrelor group were not significantly different than clopidogrel group.12) As PHILO was a much smaller study involving only 801 patients, it is necessary to closely examine the efficacy and safety of these two drugs in the East Asian population. In this study, we conducted a meta-analysis to evaluate and compare the efficacy and safety of ticagrelor and clopidogrel based on existing studies on East Asian patients with ACS.
1. Search strategy
We searched PubMed, Embase and the Cochrane Central Register of Controlled Trials for all published articles. We used the terms of (“acute coronary syndrome”OR “acute coronary syndromes”OR “ACS”OR “unstable angina”OR “UA”OR “non-ST-segment elevation”OR “non-ST segment elevation”OR “non ST-segment elevation”OR “non-ST-elevation”OR “non ST-elevation”OR “non-ST elevation”OR “NSTEMI”OR “ST-segment elevation”OR “ST segment elevation”OR “ST-elevation”OR “ST elevation”OR “STEMI”OR “myocardial infarction”OR “myocardial infarctions”OR “MI”OR “AMI”OR “non-ST-elevation acute coronary syndrome”OR “non-ST-elevation acute coronary syndromes”OR “NSTE-ACS”OR “Percutaneous coronary intervention”OR “PCI”) AND (“ticagrelor”OR “Brilinta”OR “Brilique”OR “Possia”OR “AZD6140”) AND (“clopidogrel”OR “Plavix”) in each database. One article in Chinese was available after translating into Korean.
2. Inclusion and Exclusion criteria
Two investigators performed searching and selecting articles independently. We resolved disagreements through consulting the articles. Inclusion criteria were: (1) clinical trials or cohort studies consisted of East Asian patients (Korean, Japanese, Taiwanese, and Chinese etc.); (2) patients with ACS were treated with clopidogrel or ticagrelor; (3) doses of clopidogrel or ticagrelor followed the ACC/AHA guidelines; (4) studies evaluated outcomes such as composite of MI, stroke and cardiovascular (CV) death as efficacy endpoint and major bleeding as safety endpoint. Refworks 2.0 (RefWorks-COS, ProQuest LLC), a web-based data manager program, was used for selection and assessment of data quality.
3. Groups and Endpoints
We established two groups: patients treated with clopidogrel and patients treated with ticagrelor. To evaluate the differences of efficacy and safety of two drugs, we determined the composite MI, stroke and CV death as the efficacy endpoint, and major or minor bleeding as safety endpoint. Major bleeding was defined as bleeding that led to clinically significant disability (e.g., intraocular bleeding with permanent vision loss) or bleeding either associated with a drop in the hemoglobin level of at least 3.0 g per deciliter but less than 5.0 g per deciliter or requiring transfusion of 2 to 3 units of red cells. Minor bleeding was defined as any bleeding requiring medical intervention but not meeting the criteria for major bleeding. These endpoints were based on the criteria defined by the PLATO trial.6)
We categorized the studies according to the study design (i.e. randomized controlled trials (RCTs) and non-RCTs (cohort or observation studies). Then, we extracted data from each study, including first author, publication year, information on study subjects (type of ACS, age, race, sex, etc), number of treatment group, drug dose, study design, inclusion criteria, exclusion criteria, and primary efficacy and safety endpoint outcomes.
Using the extracted data, we assessed the risk of biases for RCTs using Revman 5.3 (The Cochrane Collaboration, Copenhagen, Denmark) and the guidance in the Cochrane Handbook, version 5.1.0. For non-RCTs, we estimated the biases according to Newcastle-Ottawa scale (NOS). We allocated the extracted outcomes as dichotomous frequency data. We conducted the meta-analysis based on the pooled odds ratios (OR) and 95% confidence intervals (CIs) in random-effects models. We evaluated heterogeneity among studies by Q test and I2 statistics.
1. Characteristics of included studies
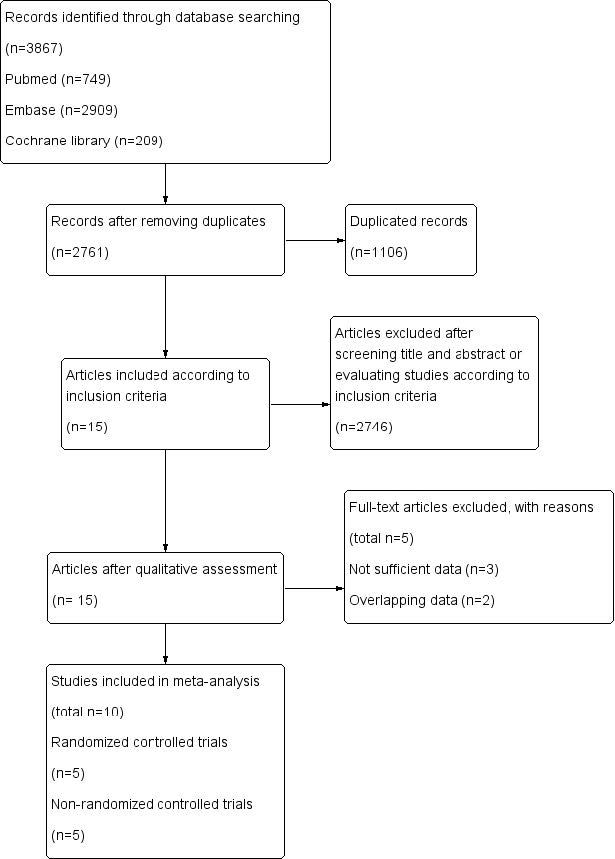
A total of 3,867 studies retrieved by databases (PubMed, EMBASE, and Cochrane Library) were identified. After screening for the titles and abstracts, 2,761 duplicated articles and 2,746 articles that did not meet the inclusion criteria were excluded. Among the available full-text articles, 5 articles were excluded for overlapping and inappropriate data. A total of 10 studies were included in our meta-analysis (Fig. 1).12-21) Among them, 5 studies were RCTs12-16) and rest of the studies were non-RCTs.17-21)
Baseline characteristics of included RCTs and non-RCTs are described in Table 1 and 2. Overall, in RCTs, follow-up period was approximately 12 months with the exception of one RCT which was 6 months. The average age of included patients was in the mid-60s except for one study. Of the 5 non-RCT studies, four were cohort studies. Follow-up periods of non-RCTs were variable from one to 12 months.
2. Risk of bias assessment
In the bias assessment of RCTs, there were 7 categories of bias: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias. Among RCTs, in the reporting of the allocation concealment, four studies were low risk, whereas one study was considered to have unclear risk. The study had unclear risk in the reporting blinding of participants and the outcome assessment.14) And two studies were considered high risk because of funding from pharmaceutical companies. Otherwise in non-RCTs, there were three categories of bias: selection, comparability, and outcome bias. Among the cohort studies, there were no studies with high risk of bias.
3. Outcomes of meta-analysis
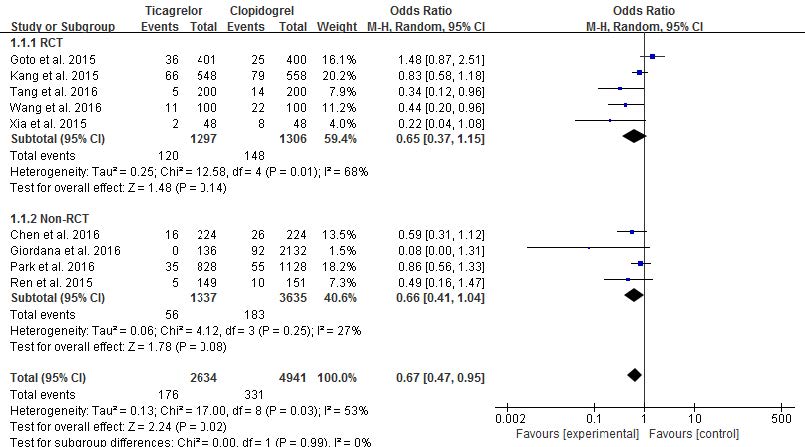
In this study, efficacy and safety endpoints of each RCT and non-RCT groups are described in Table 3. The composite of MI, stroke and CV death was reported in a total of 9 studies: 5 studies in RCT group and four studies in non-RCT group. Each group had low heterogeneity according to random-effects models (p=0.99, I2=0%). In the RCT group, among a total of 2,603 patients, 268 patients experienced the composite of MI, stroke and CV death: 120 patients in ticagrelor group and 148 patients in clopidogrel group. Heterogeneity was somewhat demonstrated (p=0.01 and I2=68%). In the non-RCT group, no significant difference was shown between ticagrelor and clopidogrel with low heterogeneity (p=0.25, I2=27%). In RCT groups, the composite of MI, stroke and CV death of ticagrelor was not significantly different from clopidogrel (9.25% vs. 11%, OR 0.65, 95% CI 0.37-1.15, p=0.14, Fig. 2). Likewise, in non-RCT groups, the incidence of the composite of MI, stroke and CV death in the ticagrelor group was not significantly different from that of clopidogrel group (4.19% vs. 5.03%, OR 0.66, 95% CI 0.41-1.04, p=0.08, Fig. 2). When the results of RCTs and non-RCTs were combined, the efficacy outcome occurred in 6.68% in the ticagrelor group compared to 6.70% in the clopidogrel group (OR 0.67, 95% CI 0.47-0.95, p=0.02).
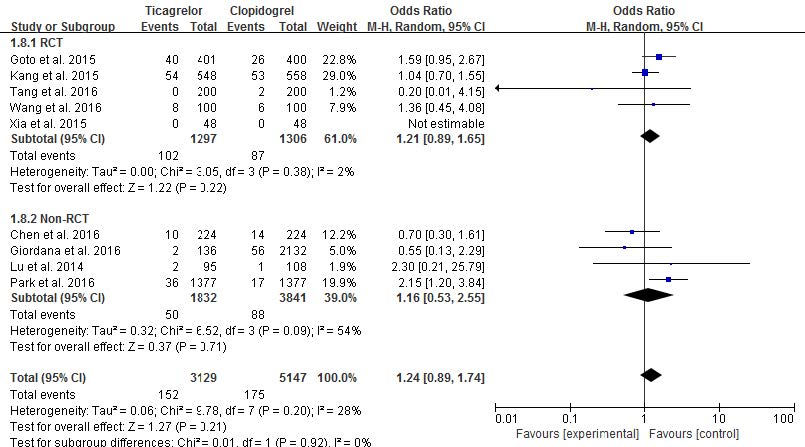
Nine studies reported risk of major bleeding with no heterogeneity (p=0.20, I2=28%): five studies in the RCT group and four studies in the non-RCT group. And no differences in heterogeneity were observed between the RCT and non-RCT groups (p=0.92, I2=0%). There was low heterogeneity in RCT groups (p=0.38, I2=2%). Among the RCTs, the incidence of major bleeding risk was similar between ticagrelor and clopidogrel (7.86% vs. 6.66%, OR 1.21, 95% CI 0.89-1.65, p=0.22, Fig. 3). In one study,14) because there was no incidence of major bleeding, the weight and odds ratio were not estimated. Likewise, no significant differences in major bleeding were observed between ticagrelor and clopidogrel in non-RCTs (2.29% vs. 2.72%, OR 1.16, 95% CI 0.53-2.55, p=0.71, Fig. 3). Major bleeding rates of 4.86% in ticagrelor and 3.40% in clopidogrel were observed when all nine studies were combined, but the difference was not significant (OR 1.24, 95% CI 0.89-1.74, Fig. 3). As a result, ticagrelor has a similar risk of major bleeding as clopidogrel in East Asian patients with ACS.
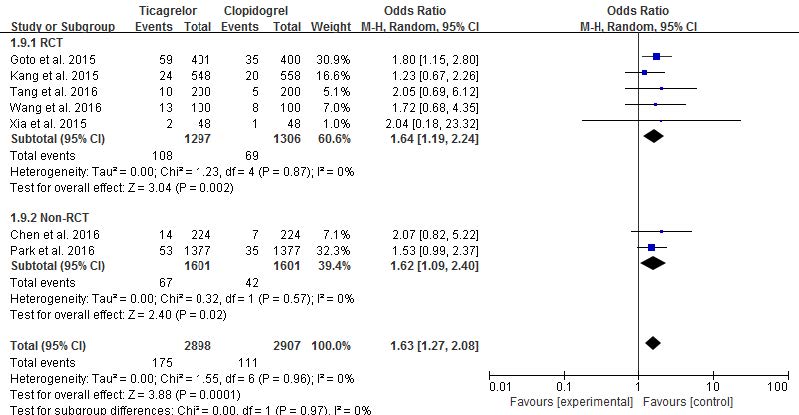
For minor bleeding, seven studies were analyzed (five studies in RCT and two studies in non-RCT). There was no heterogeneity and no differences between RCT and non-RCTs (p=0.96, I2=0%, p=0.97, I2=0%, respectively). In RCTs, the risk of minor bleeding was significantly higher in ticagrelor than clopidogrel (8.33% vs. 5.28%, OR 1.64, 95% CI 1.19-2.24, p=0.002, Fig. 4). This trend also was shown in non-RCTs (4.8% vs. 2.62%, OR 1.62, 95% CI 1.09-2.40, p=0.02, Fig. 4). In total, the risk of minor bleeding in ticagrelor was significantly higher than that of clopidogrel (6.04% vs. 3.82%, OR 1.63, 95% CI 1.27-2.08, p=0.0001, Fig. 4). Therefore, the risk of minor bleeding was significantly increased in ticagrelor compared to clopidogrel in East Asian patients with ACS.
|
Fig. 1 Flow diagram of study selection |
|
Fig. 2 Risk comparison of the composite of MI, stroke and CV death in randomized controlled trials and non-randomized controlled trials (random-effects models) |
|
Fig. 3 Risk comparison of major bleeding in randomized controlled trials and non-randomized controlled trials (random-effects models) |
|
Fig. 4 Risk comparison of minor bleeding in randomized controlled trials and non- randomized controlled trials (random-effects models) |
|
Table 1 Characteristics of included randomized controlled trials |
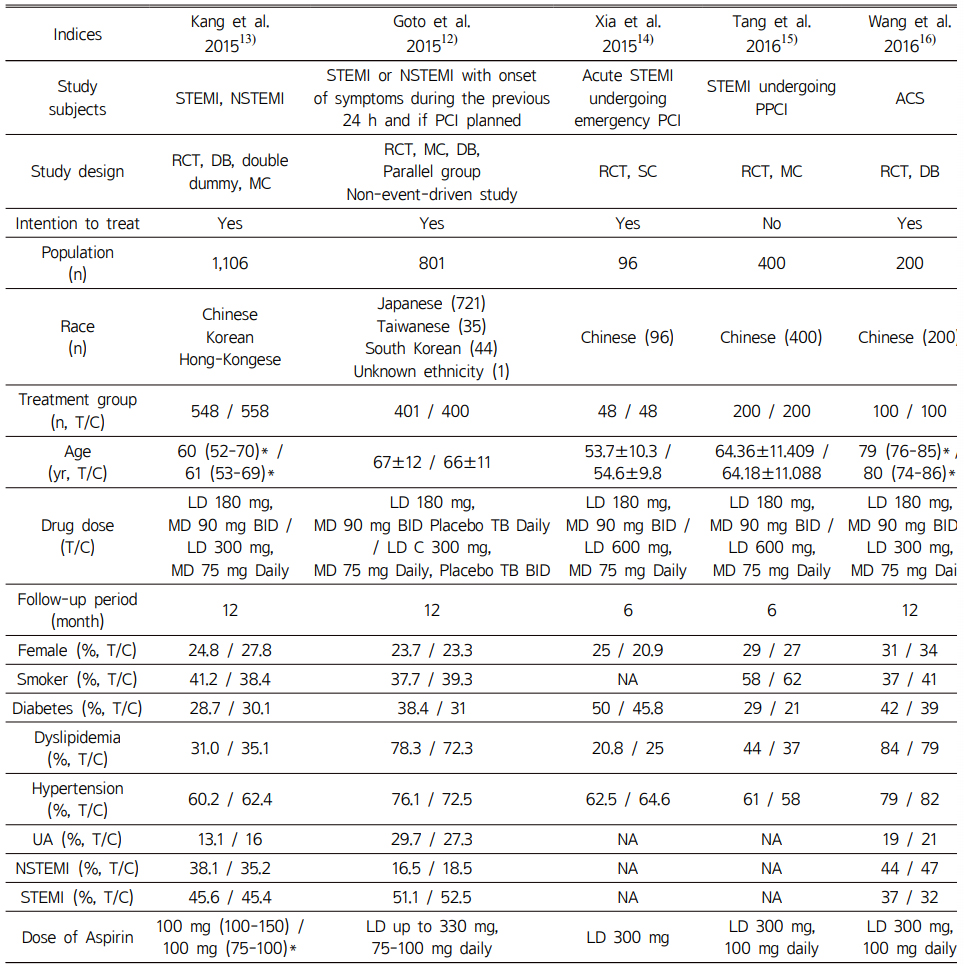
*interquartile range; T/C, Ticagrelor/Clopidogrel; yr, year; ACS, acute coronary syndromes; RCT, randomized controlled trials; MC, multi-center study; SC, single-center study; LD, loading dose; MD, maintenance dose; UA, unstable angina; NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; PPCI, pre-percutaneous coronary intervention; PCI, percutaneous coronary intervention; NA, not available |
|
Table 2 Characteristics of included non- randomized controlled trials |
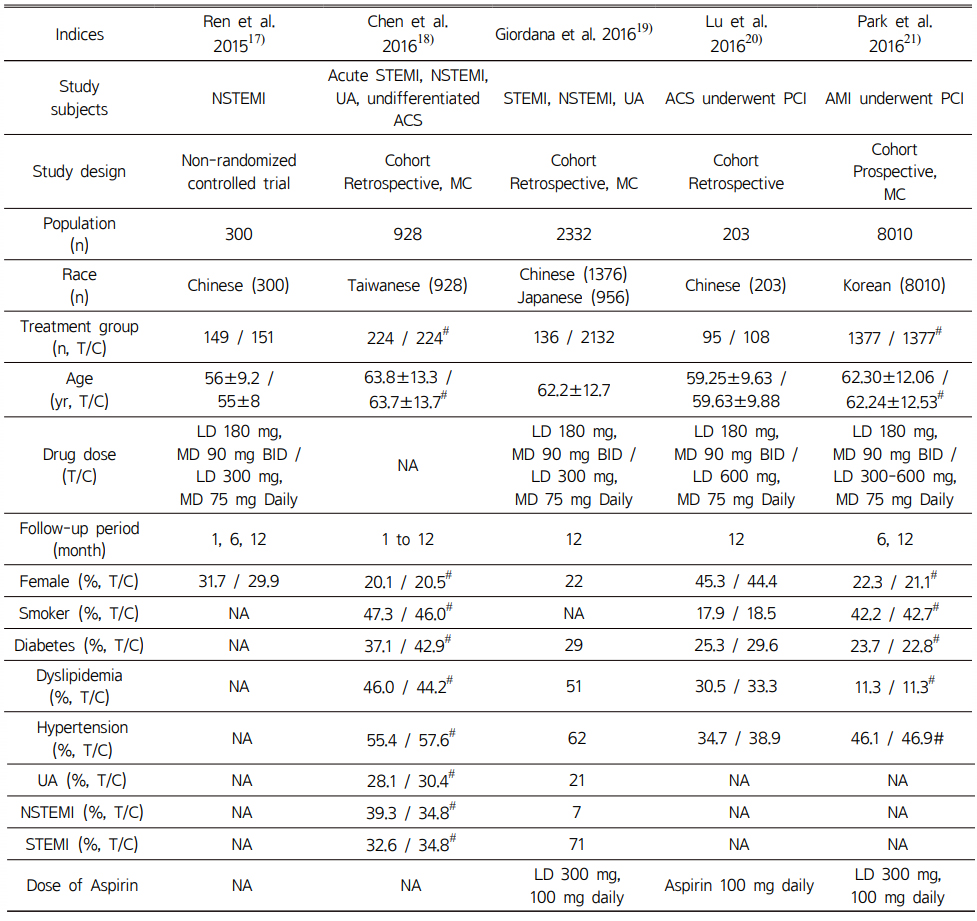
#, after propensity score matching; T/C, Ticagrelor/Clopidogrel; yr, year; ACS, acute coronary syndromes; MC, multi-center study; SC, single-center study; LD, loading dose; MD, maintenance dose; UA, unstable angina; NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; NA, not available |
|
Table 3 Efficacy and safety endpoints of included randomized controlled trials (A) and non- randomized controlled trials (B) |
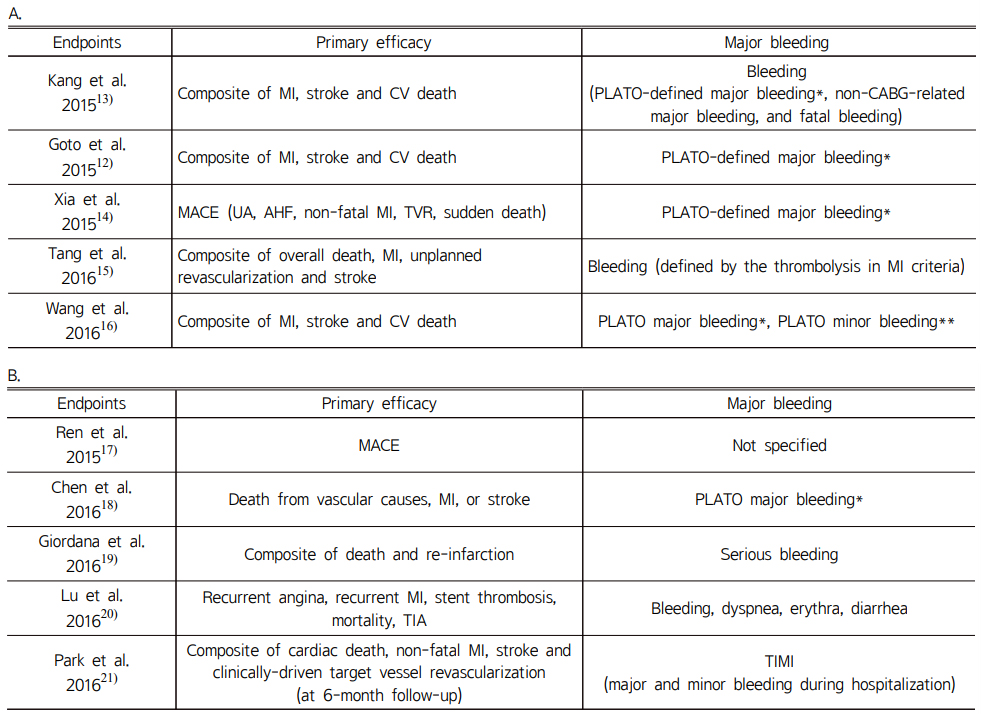
A |
Many randomized trials proved that the use of low dose aspirin helps to reduce the rate of mortality and risk of MI in ACS patients.22) Surprisingly, in CURE trial, adding a P2Y12 inhibitor such as clopidogrel showed significant lower event in the composite of death, MI and stroke, compared with aspirin alone.23) Many trials consistently demonstrated that following PCI, DAPT reduces activation of platelet and ischemic or thrombotic complications.24-26) As such, DAPT has established itself as the standard strategy for ACS patient treatment.
Ticagrelor, which was developed later than clopidogrel, has more potent and consistent antiplatelet effects than clopidogrel, and it acts directly on the P2Y12 receptor without being affected by cytochrome P450 enzymes such as CYP2C19. However, ticagrelor has to be taken twice a day, whereas clopidogrel is only taken once a day, which can be a disadvantage in terms of adherence.
In this study, we evaluated the efficacy and safety of clopidogrel and ticagrelor in patients with ACS through a meta-analysis of RCTs and non-RCTs in East Asian population. When the composite of MI, stroke and CV death as efficacy endpoint was compared, ticagrelor had significantly reduced event rates compared to clopidogrel in East Asians. While no differences were observed in major bleeding, the risk of minor bleeding was increased in ticagrelor compared to clopidogrel in this meta-analysis. The results of our study are similar to the PLATO trial in that ticagrelor had better efficacy outcomes and similar major bleeding rates. In the PLATO trial, the rates of major or minor bleeding was significantly increased in the ticagrelor group (16.1% vs. 14.6%, HR 1.11, 95% CI 1.03-1.20), but rates of major or minor bleeding was not significantly different between ticagrelor and clopidogrel (11.4% vs. 10.9%, HR 1.05, 95% CI 0.96-1.15).6) On the other hand, our study results differ from the PHILO study conducted in East Asians (Japanese, Korean, Taiwanese, etc), where the primary efficacy and safety of ticagrelor and clopidogrel groups were not significantly different (9.0% vs. 6.3%, HR 1.47, 95% CI 0.88-2.44).12) TICAKOREA, another study recently conducted in Koreans with ACS evaluated the bleeding risk between ticagrelor and clopidogrel, found that significantly increased bleeding was observed in the ticagrelor group (11.7%) compared to clopidogrel group (5.3%) at 12 months (HR 2.26, 95% CI 1.34-3.79, p=0.002).27) While not adequately powered to examine the efficacy endpoint, incidence of death from CV causes, MI, or stroke was not significantly different between the ticagrelor and clopidogrel groups (9.2% vs. 5.8%, HR 1.62, 95% CI 0.96-2.74, p=0.07).27) While both PHILO (n=801) and TICAKOREA (n=800) had relatively smaller number of included patients, it is interesting that these studies, which included only East Asians, resulted in a trend towards increased incidence of primary efficacy endpoint in the ticagrelor group, which is the opposite of the findings from PLATO. It is evident that larger RCTs are needed to evaluate the clinical efficacy and safety of ticagrelor compared to clopidogrel in East Asian patients with ACS.
Among RCTs, ticagrelor significantly reduced the incidence of CV related events in two studies, Tang et al.15) and Wang et al.16) Tang et al. suggested that the characteristics of race and older age could affect platelet inhibition of ticagrelor.15) Wang et al.16) and Xia et al.14) designed the trials similarly, but the results were different. While maintenance dose, duration of intervention, and baseline characteristics of included patients were similar, there were differences in the size of the trials and the loading dose of clopidogrel. Such differences are considered to result in inconsistent outcomes between the two studies.
Most of the studies included in the meta-analysis used standard dose of the drugs: 600 mg loading dose of clopidogrel followed 75 mg/day and 180 mg loading dose of ticagrelor followed 90 mg twice a day. But when it comes to recent studies, using half or one quarter dose of ticagrelor presented higher efficacy in Chinese.28) In many previous pharmacodynamic studies, low dose ticagrelor was found to have a larger antiplatelet effects compared to clopidogrel.28-33) Larger size studies evaluating clinical endpoints of the most optimal dose of ticagrelor in East Asian ACS patients are necessary.
Limitation of this meta-analysis is that the number of included articles is insufficient. In addition, the size of studies, including RCT and non-RCT trials, was smaller than that of PLATO trial. In case of non-RCTs, some, but not many studies with relatively large size of patient population was included. If studies of larger scale are conducted in the future, more meaningful results could be analyzed in East Asian patients.
We conducted a meta-analysis with data from 5 RCTs and 5 non-RCTs to investigate whether ticagrelor was more effective and safer than clopidogrel in East Asians. The result of our meta-analysis showed that ticagrelor significantly lowered the risk of composite of MI, stroke and CV death in the ticagrelor group, whereas the risk of major bleeding was similar to clopidogrel. However, the risk of minor bleeding in ticagrelor occurred more frequently than that of clopidogrel in East Asians.
- 1. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014 Dec 23;130(25):e344-426.
-

- 2. Cardiovascular diseases. World Health Organization. Available at: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed May 15, 2021).
- 3. Ohira T, Iso H. Cardiovascular disease epidemiology in Asia: an overview. Circ J 2013;77(7):1646-52.
-

- 4. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016;68(10):1082-1115.
-

- 5. Bauer, Shawn M. ADP receptor antagonists as antiplatelet therapeutics. Expert Opin Emerg Drugs 2003;8(1):93-101.
-

- 6. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045-57.
-

- 7. Jernberg T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J 2006;27:1166-73.
-

- 8. Goldstein JA, Ishizaki T, Chiba K, et al. Frequencies of the defective CYP2C19 alleles responsible for the mephenytoin poor metabolizer phenotype in various Oriental, Caucasian, Saudi Arabian and American black populations. Drug Metab Dispos 2010;38: 92-99.
- 9. Hwang SJ, Jeong YH, Kim IS, et al. The cytochrome 2C19*2 and *3 alleles attenuate response to clopidogrel similarly in East Asian patients undergoing elective percutaneous coronary intervention. Thromb Res 2011;127(1):23-8.
-

- 10. Storey RF, Husted S, Harrington RA, et al. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol 2007;50:1852-6.
-

- 11. Husted S, Emanuelsson H, Heptinstall S, et al. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. Eur Heart J 2006;27:1038-47
-

- 12. Goto S, Huang CH, Park SJ, et al. Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome – randomized, double-blind, phase III PHILO study. Circ J 2015; 79(11):2452-60.
-

- 13. Kang HJ, Clare RM, Gao R, et al. Ticagrelor versus clopidogrel in Asian patients with acute coronary syndrome: A retrospective analysis from the Platelet Inhibition and Patient Outcomes (PLATO) Trial. Am Heart J 2015;169(6):899-905.e1.
-

- 14. Xia JG, Qu Y, Hu SD, et al. Midterm follow-up outcomes of ticagrelor on acute ST segment elevation myocardial infarction undergoing emergency percutaneous coronary intervention. Beijing Da Xue Xue Bao 2015;47(3):494-8.
- 15. Tang X, Li R, Jing Q, et al. Assessment of Ticagrelor Versus Clopidogrel Treatment in Patients With ST-elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. J Cardiovasc Pharmacol 2016;68(2):115-20.
-

- 16. Wang H, Wang X. Efficacy and safety outcomes of ticagrelor compared with clopidogrel in elderly Chinese patients with acute coronary syndrome. Ther Clin Risk Manag 2016;12:1101-5.
-

- 17. Ren Q, Ren C, Liu X, et al. Ticagrelor vs. clopidogrel in non-ST-elevation acute coronary syndromes. Herz 2016;41(3):246-9.
-

- 18. Chen IC, Lee CH, Fang CC, et al. Efficacy and safety of ticagrelor versus clopidogrel in acute coronary syndrome in Taiwan: A multicenter retrospective pilot study. J Chin Med Assoc 2016;79(10):521-30.
-

- 19. Giordana F, Montefusco A, D’Ascenzo F, et al. Safety and effectiveness of the new P2Y12 inhibitor agents vs clopidogrel in ACS patients according to the geographic area: East Asia vs Europe. Int J Cardiol 2016;220:488-95.
-

- 20. Lu Y, Li Y, Yao R, et al. Clinical effect of ticagrelor administered in acute coronary syndrome patients following percutaneous coronary intervention. Exp Ther Med. 2016;11(6):2177-2184.
-

- 21. Park KH, Jeong MH, Ahn Y, et al. Comparison of short-term clinical outcomes between ticagrelor versus clopidogrel in patients with acute myocardial infarction undergoing successful revascularization; from Korea Acute Myocardial Infarction Registry-National Institute of Health. Int J Cardiol 2016;215:193-200.
-

- 22. Antithrombotic Trialists’Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71-86.
-

- 23. Yusuf S, Zhao F, Mehta SR, et al. Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494-502.
- 24. Schömig A, Neumann F-J, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med 1996;334:1084-1089.
-

- 25. Rupprecht HJ, Darius H, Borkowski U, et al. Comparison of antiplatelet effects of aspirin, ticlopidine, or their combination after stent implantation. Circulation 1998;97:1046-1052.
-

- 26. Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. N Engl J Med 1998;339:1665-1671.
-

- 27. Park DW, Kwon O, Jang JS, et al. Clinically significant bleeding with ticagrelor versus clopidogrel in Korean patients with acute coronary syndromes intended for invasive management: a randomized clinical trial. Circulation 2019;140(23):1865-1877.
-

- 28. He M, Liu B, Sun D, et al. One-quarter standard-dose ticagrelor better than standard-dose clopidogrel in Chinese patients with stable coronary artery disease: A randomized, single-blind, crossover clinical study. Int J Cardiol 2016; 215: 209-213.
-

- 29. Guo LZ, Kim MH, Jin CD, et al. Comparison of pharmacodynamics between low dose ticagrelor and clopidogrel after loading and maintenance doses in healthy Korean subjects. Platelets 2015;26(6):563-9.
-

- 30. Hiasa Y, Teng R, Emanuelsson H. Pharmacodynamics, pharmacokinetics and safety of ticagrelor in Asian patients with stable coronary artery disease. Cardiovasc Interv Ther 2014; 29: 324.332.
-

- 31. Li H, Butler K, Yang L, et al. Pharmacokinetics and tolerability of single and multiple doses of ticagrelor in healthy Chinese subjects: an open-label, sequential, two-cohort, single-centre study. Clin Drug Investig 2012;32(2):87-97.
-

- 32. Liu GZ, Zhang S, Sun DH, et al. Half-dose ticagrelor versus high-dose clopidogrel in reducing platelet reactivity in acute coronary syndrome patients with high on-clopidogrel platelet reactivity (divide study). Eur J Clin Pharmacol 2019;75(8):1059-1068.
-

- 33. Xue HJ, Shi J, Liu B, et al. Comparison of half- and standard-dose ticagrelor in Chinese patients with NSTE-ACS. Platelets 2016;27(5):440-5.
-

 This Article
This Article
-
2021;7(1):20-29
Published on May 31, 2021
- Received on May 26, 2021
- Revised on May 31, 2021
- Accepted on May 31, 2021
 Services
Services
Shared
 Correspondence to
Correspondence to
- Yun Jeong Lee
-
Rm 207, College of Pharmacy, Dankook University, 119, Dandae-ro, Dongnam-gu, Cheonan, Chungnam, 31116, Republic of Korea
Tel: +82-41-550-1445, Fax: +82-41-559-7899 - E-mail: yunlee@dankook.ac.kr







